November 16, 2022 | Advocacy, Covid-19, Treatments
Important notice for MS patients regarding EvusheldTM.
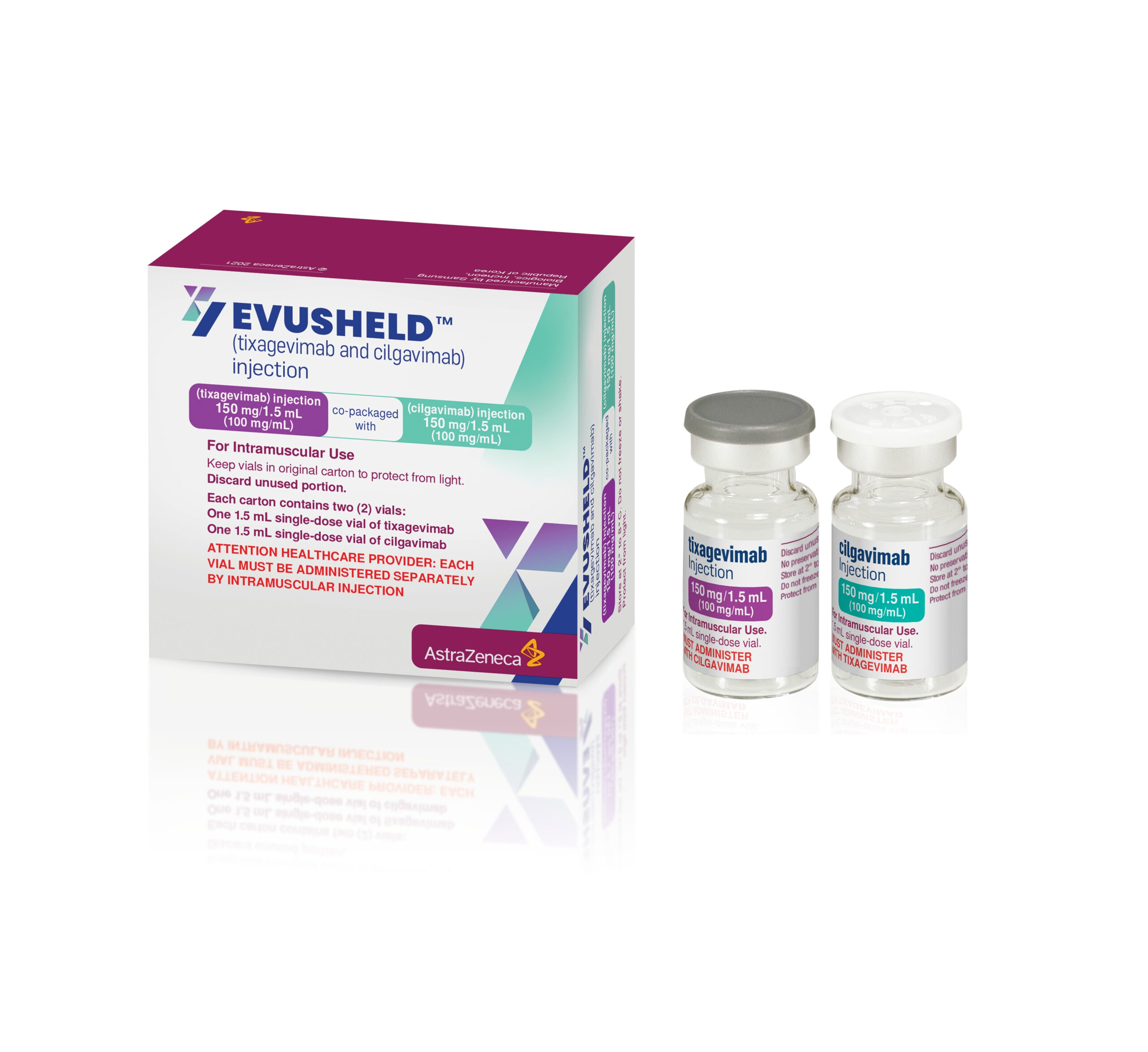
EvusheldTM is a pre-exposure prophylaxis, which has shown positive results for reducing the impacts of COVID-19 on those who may lowered immunity against COVID 19 due to their MS treatments. Evusheld is NOT a vaccine but consists of two synthetic antibodies (made in the laboratory) which help boost the immune system before infection takes place to fight off infection.
Following conversations with our specialist Neurology colleagues at Te Whatu Ora Waitara Canterbury, (formerly Canterbury DHB), Multiple Sclerosis NZ is sharing this information to assist you in deciding whether this medication may benefit you.
What is Evusheld and how does it work?
EvusheldTM contains 2 medicines – tixagevimab and cilgavimab (300mg each). They belong to a group of medicines called anti-SARS-CoV-2 monoclonal antibodies. When taken together they bind to the virus that causes COVID-19 infection (SARS-CoV-2) and prevent it from infecting healthy cells in your body.
EvusheldTM may protect people from getting, or becoming very sick from, COVID-19. It may also reduce the risk of being admitted to hospital.
How is Evusheld administered?
Your GP or nurse can administer Evusheld. It is given as 2 injections into a muscle, usually the buttocks, one after the other. You will be asked to wait for a short period for observation as some people can have an allergic reaction.
The medication benefits appear to last for up to 6 months and repeat doses may be required for ongoing protection.
Who may Evusheld be useful for?
Evusheld is given to people to protect them from getting COVID-19 or from becoming very sick if they do get COVID-19. It may reduce their risk of being admitted to hospital. The Ministry of Health have recommended Evusheld for people who may be immunocompromised or those who may be at risk of an inadequate response to SARS-CoV-2 vaccines. This may include people who, in the last 12 months, have been treated with Ocrelizumab, (Ocreveus) Rituximab, alemtuzumab or sphingosine1 -phosphate receptor modulator (Fingolimod) or those who, in the last 6 months have received cyclophosphamide.
Generally, it is not used when a person has COVID-19. If you are taking any of the above medications and have been diagnosed with COVID-19 in the last 5-7 days, you may be eligible for special antiviral medications and should contact your GP to discuss. (Link to the Paxlovid data)
Evusheld is given to people who have trouble making antibodies to fight disease, e.g, if you have a severely weakened immune system (immunocompromised). This mainly includes people with conditions requiring bone marrow or organ transplants We know however that some people with MS who are on the above medications may have made a weakened response to the COVID-19 virus. It is however reassuring that in New Zealand, likely because of our high vaccination rates, we have no reports of people with MS on these drugs requiring hospitalisation. Most reports from overseas suggest that people with severe adverse outcomes from COVID-19 were older (> 60), had other health issues and did not have timely access to vaccinations.
The population included in the studies of Evusheld that showed a significant reduction (77%) in symptomatic COVID-19 (8/3441 (0.2%) in the treatment group versus 17/1731 (1.0%) of the placebo group) were largely unvaccinated at entry to the trial and had not had prior infection with COVID-19 although all five patients who developed severe Covid-19 including two deaths were in the placebo group. The study appears to have included few people with MS on disease modifying agents.
Is Evusheld another Covid-19 Vaccine?
No. Evusheld is NOT a vaccine. It consists of synthetic antibodies (made in the laboratory) which help boost your immune system to fight off infection. Rather than fighting off the virus, it essentially “soaks it up”. These antibodies are derived from antibodies isolated from people infected with SARS-CoV-2 so the antibodies are most effective against the variants that were circulating at the time the medicine was made. It is still to be determined whether Evulsheld will be effective against newer SARS-CoV-2 variants.
For most people, vaccination is recommended as the best way to protect yourself against COVID-19.
What do MS Specialists recommend?
Our specialist neurological colleagues have reviewed the trial data in collaboration with infectious disease specialists. Following this review the data suggests Evusheld is a useful addition to our toolbox in protecting PwMS against severe COVID-19. In our specialists’ opinions, there may be particular benefits for PwMS, who are not vaccinated or had their vaccines after starting treatment with disease modifying medications, those who are older > 60 or who have other co-morbidities such as hypertension. There is also evidence that the protection offered by Evusheld is more effective than having had the COVID virus itself and we would also recommend it in those who have not yet tested positive for COVID-19.
As such it is recommended that patients discuss with their health care provider whether it would be beneficial in their individual circumstance
Is Evusheld right for everyone?
All patients receiving Evusheld must be ≥12 years old, ≥ 40kg and not have had a recent (within 8 days) known COVID-19 infection.
Sometimes a medicine is not suitable for a person with certain health conditions or on specific medicines. When considering Evusheld you should tell your healthcare provider if you have:
You should also ensure your healthcare provider is aware of all medicines and supplements you are taking as some may be contraindicated.
There isn’t a lot of research on the impact of Evusheld on pregnancy and breastfeeding. If you are pregnant, planning a pregnancy or breastfeeding, talk to your doctor about the potential risks and benefits. Although the risks are likely to be small, consultation with an obstetric physician is advised.
Evusheld is designed for those who may still remain vulnerable to Covid despite vaccination. However, it is important you tell your healthcare provider before using Evusheld which vaccinations you have already received. It is possible that Evusheld may reduce your body’s immune response to a COVID-19 vaccine.
Are there any side effects?
As with all medicines side effects can happen. Contact your healthcare team or Healthline on 0800 611 116 immediately if you notice any side effects and tell them you are taking Evusheld. You can also report side effects to medicines to CARM (Centre for Adverse Reactions Monitoring).
Prior to vaccination all patients should be asked to give informed consent to receive the medication.
To monitor for reactions, patients should be observed by the nurse or doctor on site for a short period of time.
In general, EvusheldTM is well tolerated, however common and uncommon side effects include:
Common: may affect up to 1 in 10 people.
These are usually mild and go away with time or when the medicine is stopped. Tell your healthcare provider if these side effects cause you problems or don’t go away.
Uncommon, rare and potentially serious side effects: may affect up to 1 in 100 people
Contact your healthcare team or Healthline on 0800 611 116 immediately if you notice these side effects and tell them you are taking Evusheld.
Further information:
Information Sheet from Te Whatu Ora Waitara Canterbury: Department of Neurology
Evusheld | Health Navigator NZ
Evusheld Patient Information Sheet – Canada
COVID-19: Advice for all health professionals | Ministry of Health NZ
New Zealand’s COVID-19 treatments portfolio – Pharmac | New Zealand Government
Evesheld Medsafe Datasheet (medsafe.govt.nz)