
May 30, 2024 | Life with MS, MS Voice, My Story, News, Newsletter, Treatments
Welcome to the 2024 World MS Day Special Edition of MS Voice – your trusted source for the latest updates, information and inspiring stories from Multiple Sclerosis New Zealand. World MS Day is an international awareness day for everyone affected […]

May 30, 2024 | Advocacy, Treatments
At MSNZ we would like to understand more about patient experiences with infusion treatments. The first line Disease Modifying Treatment (DMT) options we have been successful in advocating for in NZ are considered some of the best in the world. […]
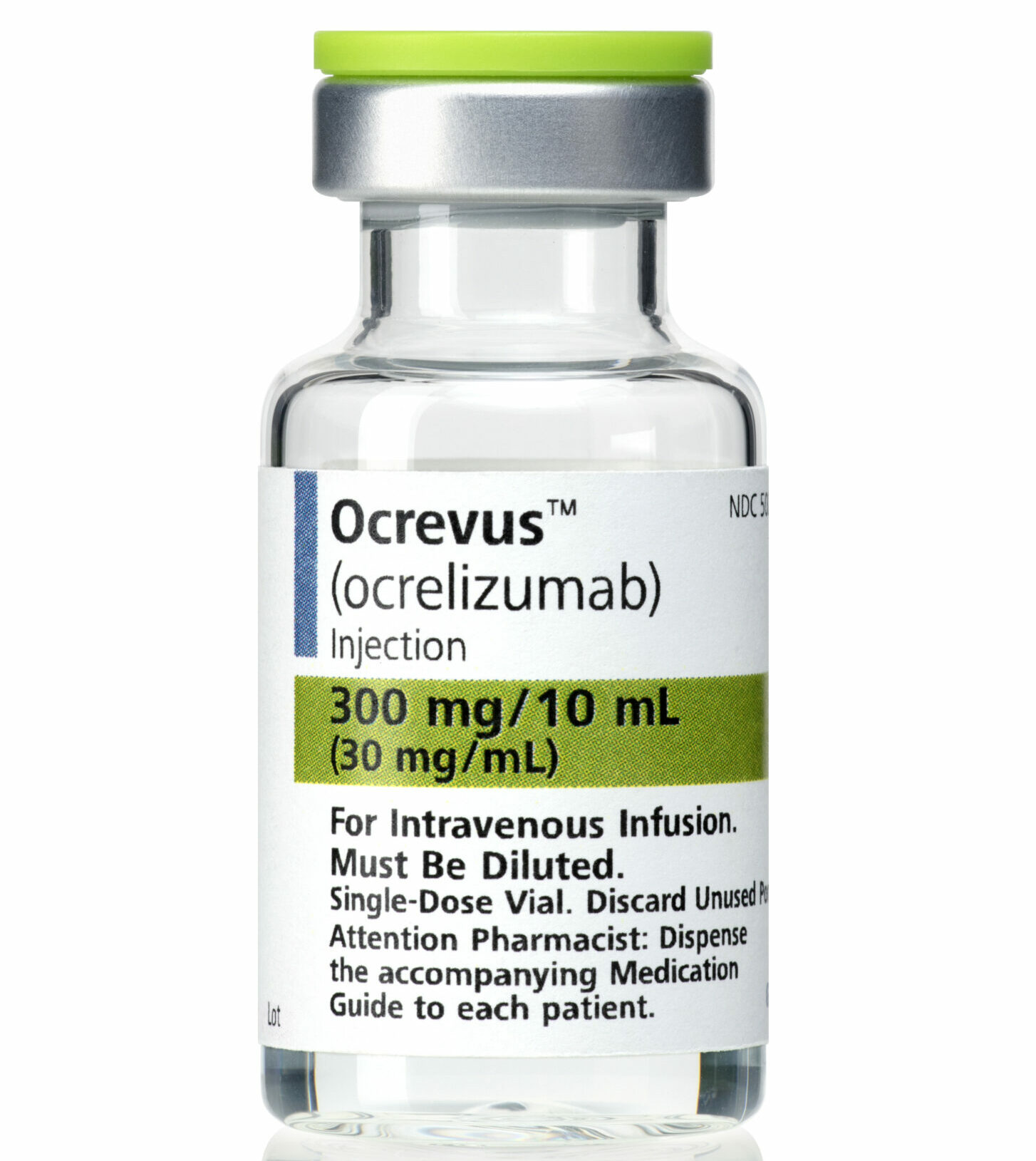
May 29, 2024 | Life with MS, News, Progressive, Treatments
On Wednesday 15 May, Multiple Sclerosis NZ raised how critical workforce shortages are impacting patients with Primary Progressive MS (PPMS) access to time sensitive disease modifying therapies. A business case prepared by clinical specialists had been under review for five […]

May 15, 2024 | Advocacy, Funding, Life with MS, Media, News, Press Release, Treatments
A celebrated Pharmac decision late last year to fund a vital drug for many Multiple Sclerosis patients has fallen flat – with dozens being turned away for treatment due to severely stretched resources across a number of services. Multiple Sclerosis […]

February 29, 2024 | Advocacy, Treatments
Pharmac announced, in February, it was looking to extend funded access to Shingrix, the new vaccination for shingles, to immunocompromised people over 18 years old. However, the proposal listed specific treatments and conditions eligible. While we were pleased to see […]